All published articles of this journal are available on ScienceDirect.
Clinical and Microbiological Effects of Systemic, Local, and Combined Application of Doxycycline in the Treatment of Periodontitis
Abstract
Introduction
Periodontitis is a complex multifactorial inflammatory disease characterized by progressive destruction of tooth-supporting tissues, including periodontal ligaments and alveolar bone. This disease contributes to tooth loss and masticatory dysfunction. It has a negative impact on patients’ nutrition, speech, aesthetics, and general health, which in turn impairs their quality of life and self-esteem. This study aimed to evaluate the clinical and microbiological effects of four different modalities, such as Scaling and Root Planning (SRP) alone, SRP with systemic doxycycline (systemic Dox), SRP with local doxycycline (local Dox), and SRP with systemic and local Dox in the treatment of periodontitis.
Methods
A clinical study of 90-day duration was conducted and included 60 patients (33 males and 27 females) diagnosed with stage II and stage III periodontal cases divided into four groups of 15 patients each: SRP alone, SRP with systemic Dox, SRP with local Dox, and SRP with systemic and local Dox. Four clinical parameters, namely, Plaque Index (PI), Gingival Index (GI), Probing Pocket Depth (PPD), and Clinical Attachment Level (CAL), as well as one microbiological parameter, such as bacterial count (Colony-Forming Units, CFUs) were recorded at baseline, day 45, and day 90. The data were documented and statistically analyzed, with a statistical significance set at p < 0.05 for all tests.
Results
The clinical and microbiological parameters improved at the 45 and 90 days of visit compared with day 0 (baseline) in all groups. Moreover, PI and GI had the most statistically significant difference (p < 0.001) in SRP with systemic and local Dox group, followed by SRP with systemic Dox group, then SRP with local Dox group and SRP only group. The most statistically significant differences in PPD, CAL, and CFUs (p < 0.001) were found in SRP with systemic and local Dox group, followed by SRP with local Dox group, then SRP with systemic Dox group, and with SRP only.
Conclusion
All the different modalities improved the clinical and microbiological parameters in patients with stages II and III periodontitis at both 45 and 90 days. Using SRP with doxycycline provided a higher improvement in clinical and microbiological parameters than that of SRP alone.
1. INTRODUCTION
Periodontitis is considered one of the main community health problems. It is considered as the principal disease in individuals and the leading cause of tooth loss and gingival harm in adults [1, 2]. The treatment of periodontitis basically depends on eliminating or minimizing dental plaque through mechanical debridement [3, 4]; however, mechanical debridement could have certain limitations, including difficulties in reaching areas like root concavities, furcations, and dental/oral sites that serve as a reservoir of bacteria [5-7]. Even after meticulous debridement, recurrent infection of the periodontal pocket due to recolonization of periodontal pathogenic bacteria could occur within 60 days, which may necessitate using adjunctive agents [7-9].
Several adjunctive agents have been used to control and treat periodontitis, together with meticulous scaling and root planning [10]. “Doxycycline” (Dox), which is an antibiotic belonging to tetracyclines, is an agent that is effective in the treatment of periodontitis [11, 12]. It is used in periodontics due to its adjunctive properties [13].
Dox is highly concentrated in periodontal tissues and fluids and has a broad antimicrobial spectrum; it is active against Gram-positive (Gm +ve) and Gram-negative (Gm –ve) bacteria [14-16] as well as against specific anaerobic bacteria that are lately known as the principal etiological reason of all systems of periodontitis; these bacteria comprise Aggregatibacter actinomycetemcomitans (Aa) and red complex bacteria–Tannerella forsythia (Tf), Porphyromonas gingivalis (Pg), and Treponema denticola (Td) [17, 18]. In addition to its antimicrobial potential, Dox has periodontal tissue regenerative properties through anticollagenase effects, reduction of osteoclast actions, inhibition of bone resorption, elevation of osteoblast actions, stimulation of bone formation, and promotion of reattachment [19-21].
Colony-forming units (CFUs or CFU/mL) are a measure of viable clonogenic cell number (bacteria, fungi, viruses, etc.). In the present study, CFUs were used to assess perio-pathogens, particularly P. gingivalis and A. actinomycetemcomitans (A.a), which are linked to the progression of periodontitis [22, 23].
A clinical trial of 3 months was conducted by Vyas et al. (2019) investigating the treatment of periodontitis with SRP alone and SRP with systemic Dox [24]. Kamble et al. (2024) counted colony-forming units (CFUs) to assess the efficacy of treatment of periodontitis using three different modalities: SRP alone, SRP with systemic Dox, and an SRP with diode laser) [25]. Other studies investigated the management of periodontitis by using local Dox only after SRP [26-28]. Previous research has shown the efficacy of several modalities in treating and reducing pocket depth in individuals post-treatment and throughout follow-up periods. Regarding the treatment of periodontitis, Dox has been used either systemically as capsules/tablets or locally as a gel. No previous study was conducted among Yemeni patients to investigate the effect of Dox. This clinical study was designed to compare the effectiveness of systemic, local, and combined (systemic and local) application of doxycycline as adjunctive to scaling and root planning in the treatment of periodontitis patients through four different modalities: Scaling and Root Planning (SRP) alone, SRP with systemic doxycycline (systemic Dox), SRP with local doxycycline (local Dox), and SRP with systematic and local together (systemic and local Dox).
2. MATERIAL AND METHODS
2.1. Study Design and Ethical Approval
Participants treated at the Department of Periodontology, Faculty of Dentistry, Sana'a University, Yemen, were recruited from December 2023 to April 2024 for this 90-day clinical and microbiological study. Clinical and microbiological investigations were carried out according to the Helsinki Declaration's ethical standards [29]. The Medical Ethical Committee of the Faculty of Medicine, Sana'a University, provided ethical approval (ref = 143 / Dec 2, 2023). All the participants signed informed consent prior to any intervention, following a thorough description of each treatment procedure and potential risks.
2.2. Sample Size Calculation
The required sample size was calculated using G*Power software (version 3.1.9.4, University of Dusseldorf) according to an earlier report where periodontitis affects 14% of the global population [30]. The effect size (d), α, and1-β (power) were 0.2, 0.05, and 0.80, respectively. The sample size was increased from 186 to 224 sites to accurately assess therapy effects, achieve representative and applicable outcomes, and track patients who withdrew during follow-ups.
2.3. Inclusion and Exclusion Criteria
The inclusion criteria were individuals of both genders, aged over 18 years, possessing a complete set of teeth (excluding third molars), diagnosed with stage II or III periodontitis, and having not undergone any periodontal treatment in the preceding 6 months. Participants with a minimum of two sites of stage II and stage III periodontal cases (the working examining sites) according to the latest periodontal disease classifications [31]. The exclusion criteria included medically compromised individuals, those who had consumed antibiotics, vitamins, or anti-inflammatory medications in the preceding three months as well as smokers and khat chewers. Individuals with a history of allergic reaction to Dox or any other tetracycline. Participants with a history of colitis due to the use of antibiotics and gastrointestinal disturbance, pregnant, lactating, and postmenopausal women, and patients under 18 years or older than 65 years.
2.4. Participants Grouping
Patients who fulfilled the necessary prerequisites and met the inclusion criteria were arranged for clinical screening, assessment, and treatments. A case sheet was designed by the researcher and revised by the supervisors. A total of 224 periodontal sites (site with the highest pocket depth value) were selected from 33 males and 27 females (60 patients) and these sites were diagnosed as stage II and stage III. The cases were divided into four groups; the first group received SRP alone and was considered as a control group, whereas the three other groups were the experimental groups and received SRP with systemic Dox, SRP with local Dox, or SRP with systematic and local Dox, respectively. Fig. (1) represents the sites and parameters as well as the treatment type.
2.5. Data Collections and Clinical Parameters Measurements
The participants' gender and age were documented. Clinical indicators were assessed at baseline and subsequently on 45th and 90th day. All evaluations and measurements were conducted by the investigator. Scores from all four areas of a single tooth were recorded to calculate PI. For the GI site, measurements were recorded at six different points from buccal and lingual, with three on each surface (mesial, middle, and distal). The highest number was recorded as the PI and GI for each site [24, 25, 28]. PI and GI were scored from 0 to 3, as described by Löe and Silness (Table 1) [32]. PPD was measured as the distance from the gingival margin to the base of the pocket at six points, three at each surface, by inserting the probe parallel to the long axis of the roots with light force during the inversion of the probe to the depth/bottom of the pocket. CAL was measured as the distance from the CEJ to the base of the pocket in six areas. The highest values were selected and recorded as PPD and CAL sites. PPD and CAL were recorded using the UNC-15 probe [AR Instrument S.A.S, France] by using a previously reported method [33].
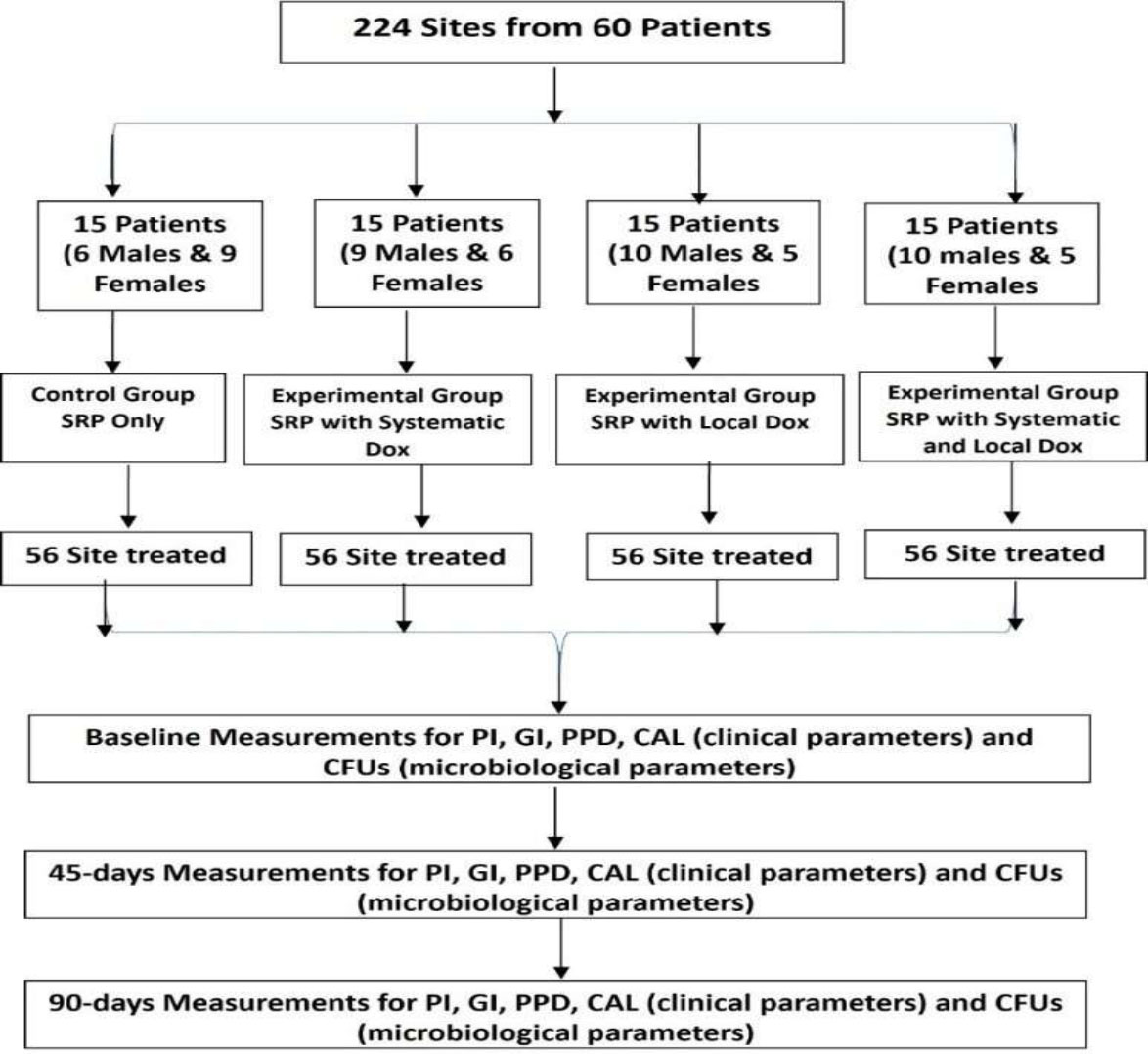
Study flowchart.
| Plaque Index | |
|---|---|
| 0 | Absence of plaque film |
| 1 | Presence of a thin film of plaque adhering to the free gingival margin and adjacent area of the assessed tooth. It may be seen in situ by using the probe on the tooth surface or after application of disclosing solution. or. |
| 2 | Presence of moderate soft plaque deposits inside the gingival pocket, or on the tooth and gingival margin which can be detected with the naked eye. |
| 3 | Plenty of soft matter within both the gingival pocket of the tooth and gingival margin. |
| Gingival index | |
| 0 | Normal and healthy gingiva |
| 1 | Mild inflammation with slight change in color and edema, but no bleeding on probing. |
| 2 | Moderate inflammation with redness, edema, and glazing, bleeding on probing. |
| 3 | Sever inflammation as marked redness, edema and ulceration, tendency toward spontaneous bleeding. |
2.6. Treatment Procedure for Participants' Preparation
Full-mouth SRP was performed on the four groups, including the control group, by using ultrasonic and hand instruments. Ultrasonic scaling was conducted using a scaler (Guilin Woodpecker Medical Instrument Co., Ltd., China) and scaler tips under continuous water irrigation. The instruments were examined before every session and replaced when worn out. Hand instrumentation of the whole dentition was performed using Gracey Curettes (AR Instrumed Pty. Ltd., Pakistan) #1-2, #3-4, #5-6, #7-8, #9-10, #11- 12 and #13-14. The teeth were scaled and root deliberate until a smooth, appropriately planned surface was achieved. Finally, all teeth were polished with a rubber cup and brushes using a prophy paste on all surfaces.
2.7. Oral Hygiene Instructions
All the study subjects were given toothbrushes (medium, Wisdom Toothbrushes Ltd., CB9 8DT Suffolk, United Kingdom) and toothpaste (silica herbal toothpaste, Dental-Kosmetik GmbH & Co. KG Katharinestr 4, 01099 Dresden, Germany). They were taught how to brush by using the modified bass technique and a pea-sized amount of toothpaste. The oral hygiene instructions were performed twice daily with tooth brushing, once daily with inter-dental cleaning and inter-dental brushes, dental floss, or triangle toothpicks.
2.8. Experimental Drugs for the Doxycycline Groups
For the systematic Dox group (systematic Dox), Dox tablets (Doxy Denk® 100 mg DENK PHARMA GmbH & Co. KG, Germany) were prescribed and used according to the manufacturer’s instructions.
Each tablet contained 115.4 mg Dox hyclate, equivalent to 100 mg Dox, as an active ingredient. Other ingredients were sodium starch glycolate, maize starch, colloidal anhydrous silica hydrogenated castor oil, lactose monohydrate, magnesium stearate, and microcrystalline cellulose. The recommended dosage of Dox was 100 mg for the treatment of periodontitis and should be given for 14 days, twice on the first day (every 12 hours 1 tablet), then once a day for the rest of the 14 days.
For the local Dox group (local Dox), Dox gel (Atridox™, Atrix Laboratories, Inc., Ft. Collins, CO, USA) was applied according to the manufacturer’s instructions. Atridox™ is a subgingival controlled-release drug that is comprised of a binary syringe mixing assembly. Syringe A contained 450 mg of the ATRIGEL® Delivery system, which is a bioabsorbable, low-viscosity polymeric design composed of 36.7% poly (DL-lactide) (PLA) liquified in a biocompatible transporter of 63.3% N-Methyl-2- Pyrrolidone (NMP). Syringe B comprised Dox hyclate, which is equivalent to 44 mg of Dox. The drug, when mixed, is a pale yellow, glutinous liquid with a concentration of 8.8% Dox hyclate. The two syringes were coupled together to mix the two components for 100 cycles. A special needle (23-gauge cannula attached to the delivery system) was used to gently insert Dox gel into the bottom of the pocket along the periodontal pocket wall [28].
The gel was lightly implanted into the pocket till the drug occupied the periodontal pocket and swamped to the gingival margin. No periodontal dressing or adhesive was applied. Upon commerce with the crevicular fluid, the liquid invention became compact. The participants were instructed not to rinse, eat, or clean their teeth within a half-hour of the drug administration, not to practice different types of oral hygiene aids at the treated extents for 168 hours, and to avoid touching these areas with toothpick, finger, or tongue [28].
For the group treated with systemic and local Dox, the steps were repeated as mentioned above and started with systemic Dox followed by local Dox.
2.9. Microbiological Evaluation
Bacterial counts (CFUs) were recorded from the 224 sites—one from each treated site and from the highest pocket depth values verified at the time of treatment (baseline) and on the 45 and 90 days. The sextant teeth were mostly isolated using cotton wool rolls and dehydrated with air. A low-volume suction was utilized to keep the chosen site dry. Subsequently, the isolation and elimination of supragingival plaque and subgingival bacteria were taken from the deepest point of the pockets by introducing a wide disinfected paper point, placed in situ for 60 seconds, collected separately, placed in 5 mL of tryptone soya broth in sterile tubes, and kept in a refrigerator at 4 °C for culture [34, 35].
The plaque samples were vortexed for 30 seconds, and 50 μL of 10-3 (1000-fold) dilutions of normal saline (0.9% NaCl) was inoculated into blood agar plates. The dilutions were done by taking 0.5 mL of the sample into 5 mL of diluents (normal saline). To obtain 10-fold dilution, we repeated the process three times to have 10-3 (1000-fold) dilution so CFUs can be counted. The serial dilutions were inoculated into blood agar media by using a 50 μL micropipette. The plates were incubated at 37 °C for 2-days. Anaerobic conditions were achieved using a simple anaerobic jar in conjunction with a standardized anaerobic process and an anaerobic gas pack. The numbers of bacteria in the culture were calculated with direct counting of the bacteria. All colonies with different sizes, colors, morphologies, and hemolytic reactions were included, and outcomes were expressed as CFUs per site for each participant and then counted manually [34, 35]. Fig. (1) shows the study flowchart as well as the site number for each group, clinical and microbiological parameters, type of treatment, and time intervals.
2.10. Statistics Analysis
Statistical analysis was conducted using SPSS Version 25 (SPSS, Version 25, Lead Technology Incorporated, USA). The values were represented as mean and standard deviation (mean ± SD) and subjected to the Kruskal–Wallis test, Wilcoxon Signed Rank test, and Mann–Whitney U test. The significance level was set at p < 0.05.
3. RESULTS
Sixty individuals (33 males and 27 females), with a mean age of 41.04±8.28 years and an age range of 27 to 61 years, completed the entire study. Fig. (2) illustrates the demographic features of the study participants.
Table 2 shows the baseline data of clinical (PI, GI, PPD, CAL) and microbiological parameters at the baseline. Based on ANOVA, all parameters were not statistically significantly different at baseline among the four groups.
Table 3 shows the inter-group comparisons (mean ± SD) for PI, GI, PPD, CAL, and CFUs at baseline and on days 45 and 90 visits for all the treated groups. In general, the assessed and evaluated values of PI, GI, PPD, and CAL decreased at 45- and 90-day visits in comparison with the baseline values. CFUs were decreased for the SRP group at 40 days and then slightly increased at 90 days; however, CFUs decreased in the other groups. ANOVA was used to assess in inter-group comparison.
Pairwise comparisons using a post-hoc test were applied among different groups (SRP alone, SRP with SDox, SRP with local Dox, and SRP with systemic Dox and local Dox). The assessed parameters (PI, GI, PPD, CAL, and CFUs) at different time intervals showed significant differences within each group at different intervals (0, 45, and 90 days; p-value < 0.05). No significant differences were recorded between 45 and 90 days for SRP alone group, and the p values for PI and CFUs were 0.112 and 0.064; no significant differences were found for GI in the SRP with the local Dox group at the same time intervals (p = 136, Table 4).
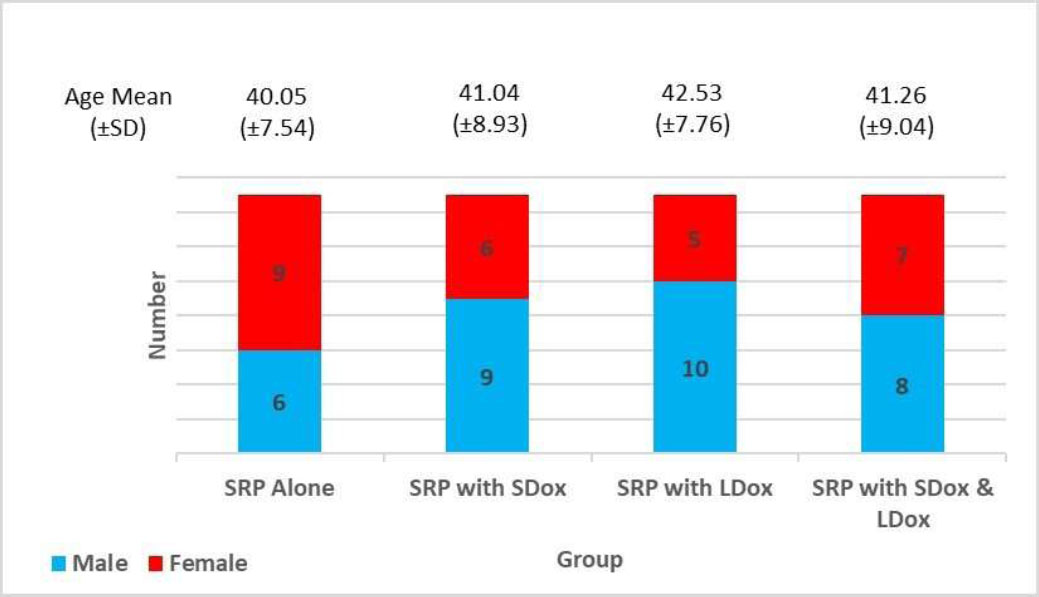
Demographic characteristics of study participants.
| Parameters | SRP Alone | SRP with Systemic Dox | SRP with Local Dox | SRP with Systemic and Local Dox | p-value |
|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD0 | ||
| Plaque index | 2.21±0.571 | 2.31±0.634 | 2.24±0.596 | 2.28±0.784 | 0.757 |
| Gingival index | 2.14±0.671 | 2.07±0.654 | 2.18±0.760 | 2.12±0.521 | 0.743 |
| Probing pocket depth | 4.41±0.541 | 4.35±0.435 | 4.46±0.541 | 4.39±0.621 | 0.633 |
| Clinical attachment level | 4.72±0.521 | 4.63±0.507 | 4.79±0.603 | 4.58±0.492 | 0.585 |
| Colony forming units | 164.02±127.44 | 176.92±118.48 | 167.07±113.74 | 173.38±130.27 | 0.322 |
Based on Table 5 and by using a post-hoc test, no significant differences were detected at baseline intervals for all the assessed groups and parameters (p > 0.050). Significant differences were recorded in the intra-group comparison of PI at 90 days between SRP alone with SRP with local Dox and SRP with both systemic and local Dox groups (p = 0.18 and 0.006). For GI, at 45 days, significant differences were recorded between SRP alone and SRP with systemic and both systemic and local Dox, SRP with systemic Dox, and SRP with local Dox group and between SRP with local Dox group and both systematic and local Dox group (p = 0.29, 0.020, 0.027, and 0.019, respectively). At 90 days, a significant difference was found between SRP alone and SRP with systemic and local Dox (p =0.049).
For PPD and CAL, significant differences were recorded at 90 days between SRP alone group and SRP with systemic and local Dox group (p = 0.031 and 0.008). Finally, for CFUs, a paired t-test showed a significant difference between SRP with the systemic Dox group and SRP with the systemic and local Dox group at 45 days (p =0.021). At 90 days, significant differences were recorded between SRP alone and the three other groups (SRP with systemic Dox, SRP with local Dox, and SRP with systemic and local Dox), with p-values of 0.039, 0.002, and 0.001, respectively. A p-value of 0.006 was documented between systemic Dox and systemic and local Dox groups (Table 5).
| Parameters | Time | SRP Alone | SRP with Systemic Dox | SRP with Local Dox | SRP with Systemic and Local Dox | p-value |
|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |||
| Plaque index | Baseline | 2.21±0.571 | 2.31±0.634 | 2.24±0.596 | 2.28±0.784 | 0.052 |
| Day 45 | 1.41±0.415 | 1.63±0.541 | 1.27±0.685 | 1.19±0.494 | ||
| Day 90 | 1.29±0.445 | 0.73±0.648 | 0.69±0.493 | 0.61±0.501 | ||
| Gingival index | Baseline | 2.14±0.671 | 2.07±0.654 | 2.18±0.771 | 2.12±0.521 | 0.031* |
| Day 45 | 1.39±0.415 | 1.03±0.442 | 1.43±0.496 | 1.01±0.431 | ||
| Day 90 | 1.1±0.565 | 0.59±0.568 | 1.06±0.793 | 0.61±0.502 | ||
| Probing pocket depth | Baseline | 4.41±0.514 | 4.35±0.435 | 4.46±0.541 | 4.39±0.621 | 0.043* |
| Day 45 | 3.88±0.498 | 3.62±0.451 | 3.75±0.496 | 3.61±0.812 | ||
| Day 90 | 3.39±0.711 | 2.95±0.681 | 3.04±0.594 | 2.91±0.405 | ||
| Clinical attachment level | Baseline | 4.72±0.521 | 4.63±0.507 | 4.79±0.603 | 4.58±0.492 | 0.062 |
| Day 45 | 4.21±0.615 | 3.98±0.622 | 4.01±0.618 | 3.83±0.712 | ||
| Day 90 | 3.76±0.752 | 3.37±0.581 | 3.36±0.745 | 3.12±0.410 | ||
| Colony forming units | Day 0 | 164.02±127.44 | 167.07±113.47 | 176.92±118.48 | 173.38±130.27 | 0.048* |
| Day 45 | 86.4±68.82 | 82.14±55.71 | 79.69±83.25 | 69.08±51.36 | ||
| Day 90 | 90.4±71.668 | 76.78±51.39 | 71.38±44.95 | 62.77±45.62 |
| Time | Pairwise Comparisons | p-value | ||||
|---|---|---|---|---|---|---|
| Plaque Index | Gingiva l Index | Probing Pocket Depth | Clinical Attachment Level | Colony Forming Units | ||
| SRP alone | Baseline vs Day 45 | 0.000* | 0.001* | 0.007* | 0.020* | 0.000* |
| Baseline vs Day 90 | 0.000* | 0.000* | 0.000* | 0.000* | 0.000* | |
| Day 45 vs Day 90 | 0.112 | 0.045* | 0.037* | 0.077* | 0.064 | |
| SRP with systemic Dox | Baseline vs Day 45 | 0.000* | 0.000* | 0.000* | 0.004* | 0.000* |
| Baseline vs Day 90 | 0.000* | 0.000* | 0.000* | 0.000* | 0.000* | |
| Day 45 vs Da 90 | 0.016* | 0.031* | 0.003* | 0.009* | 0.007* | |
| SRP with local Dox | Baseline vs Day 45 | 0.000* | 0.003* | 0.000* | 0.001* | 0.000* |
| Baseline vs Day 90 | 0.000* | 0.000* | 0.000* | 0.000* | 0.000* | |
| Day 45 vs Day 90 | 0.026* | 0.136 | 0.001* | 0.014* | 0.018* | |
| SRP with systemic and local Dox | Baseline vs Day 45 | 0.000* | 0.000* | 0.006* | 0.002* | 0.000* |
| Baseline vs Day 90 | 0.000* | 0.000* | 0.000* | 0.000* | 0.000* | |
| Day 45 vs Day 90 | 0.000* | 0.026* | 0.005* | 0.002* | 0.004* | |
| Time | Pairwise Comparisons | p-value | ||||
|---|---|---|---|---|---|---|
| Plaque Index | Gingiva l Index | Probing Pocket Depth | Clinical Attachment Level | Colony Forming Units | ||
| Baseline | Group 1 vs Group 2 | 0.688 | 0.774 | 0.732 | 0.635 | 0.810 |
| Group 1 vs Group 3 | 0.889 | 0.879 | 0.797 | 0.736 | 0.320 | |
| Group 1 vs Group 4 | 0.781 | 0.928 | 0.924 | 0.455 | 0.491 | |
| Group 2 vs Group 3 | 0.757 | 0.674 | 0.544 | 0.474 | 0.421 | |
| Group 2 vs Group 4 | 0.909 | 0.818 | 0.839 | 0.786 | 0.624 | |
| Group 3 vs Group 4 | 0.876 | 0.802 | 0.744 | 0.304 | 0.787 | |
| - | ||||||
| Day 45 | Group 1 vs Group 2 | 0.801 | 0.029* | 0.145 | 0.317 | 0.519 |
| Group 1 vs Group 3 | 0.504 | 0.812 | 0.479 | 0.381 | 0.405 | |
| Group 1 vs Group 4 | 0.197 | 0.020* | 0.281 | 0.129 | 0.007 | |
| Group 2 vs Group 3 | 0.692 | 0.027* | 0.458 | 0.895 | 0.743 | |
| Group 2 vs Group 4 | 0.376 | 0.901 | 0.967 | 0.543 | 0.021* | |
| Group 3 vs Group 4 | 0.684 | 0.019* | 0.573 | 0.465 | 0.136 | |
| - | ||||||
| Day 90 | Group 1 vs Group 2 | 0.099 | 0.051 | 0.094 | 0.132 | 0.039* |
| Group 1 vs Group 3 | 0.018* | 0.843 | 0.154 | 0.164 | 0.002* | |
| Group 1 vs Group 4 | 0.006* | 0.049* | 0.031* | 0.008* | 0.001* | |
| Group 2 vs Group 3 | 0.850 | 0.072 | 0.702 | 0.967 | 0.289 | |
| Group 2 vs Group 4 | 0.575 | 0.919 | 0.846 | 0.184 | 0.006* | |
| Group 3 vs Group 4 | 0.662 | 0.063 | 0.489 | 0.283 | 0.072 | |
4. DISCUSSION
The objective of the 90-day clinical trial was to assess and compare the efficacy of systemic Dox and local Dox alone, or the combination of systemic and local Dox as an addition to Scaling and Root Planing (SRP) in the treatment of periodontitis using four distinct modalities. The reason for the 45 days and 90 days is that doxycycline is able to decrease the count of bacteria in periodontal pockets for up to three months, and periodontal tissues require six to twelve weeks to be regenerated. Bacterial counts were determined using CFUs during the treatment of periodontitis in adult Yamani volunteers. A significant decrease in all clinical and microbiological parameters was noted across all treatment modalities. The results demonstrate that the supplementary use of Dox enhances the therapeutic efficacy of mechanical treatment. The null hypothesis is partially accepted because significant differences were detected between each group (Table 4) and within the four different modalities (SRP, systemic Dox, local Dox, and both systemic Dox and local Dox). CFUs (Table 5) were documented during and after the treatment of periodontitis in adult participants.
Periodontitis is a long-lasting multifaceted inflammatory disorder correlated to dysbiotic plaque biofilms and is characterized by advanced destruction of the tooth-supporting structures [36]. It appears in a generalized form, but it often occurs in local areas or is reduced to localized areas after phase I therapy. The cause of periodontitis is the growth of bacterial plaque on the outer surfaces of teeth, leading to free gingival tissue inflammation. Mechanical debridement, along with home-based maintenance measures (tooth brushing, flossing, and subgingival irrigation), are effective for patients with stage I or mild periodontal diseases [37, 38]. As the periodontal pocket widens, the efficacy of the patient's treatment at home and professional debridement diminishes substantially, providing local/systemic antibiotics an effective substitute [39, 40].
The systemic administration of antibiotics significantly enhances clinical and microbiological results and effectively targets all oral surfaces and fluids, yet it carries inherent side effects. To mitigate these issues, researchers have created specialized drug delivery systems [41, 42]. Locally administered antimicrobial agents are typically preferred as this method improves drug concentration at the site of action while also minimizing the overall dosage, thereby diminishing the risk of systemic side effects and enhancing patient acceptability [43]. However, the application of local antibiotics sub-gingivally is time-consuming, and some locally delivered systems are technical-dependent and expensive and require two or three follow-up visits; these factors need to be considered in the cost-benefit analysis of local antimicrobial treatments compared with systemic antimicrobial treatments [44, 45].
All assessed and evaluated clinical parameters verified showed statistically significant differences between the different recall intervals for the subjects who received scaling and root planning only. Muniz et al. and Sabatini et al. concluded that SRP alone is effective in the curing of periodontitis [46, 47]. Debridement can significantly reduce the clinical signs of periodontitis by sulcular epithelium regain, which reinforces the importance of SRP alone in periodontal therapy.
For subjects who received SRP + systemic Dox, all clinical parameters significantly differed among the baseline, 45-day, and 90-day visits. This discovery aligns with the research conducted by Vyas et al. [24]. By contrast, Yap and Pulikkotil [48] discovered that the administration of systemic Dox alongside SRP did not result in a significant improvement in clinical attachment levels or a reduction in HbA1c levels in diabetic patients with periodontitis when compared to the control group.
The results showed significant differences at all different time intervals for subjects who received SRP + Local Dox regarding all clinical parameters (with an exception for GI scores after 90-day intervals was non-significant). In this regard, a clinical trial of 90-day duration was conducted. A split-mouth design was used, and five clinical parameters, namely, PI, modified gingival index, bleeding index, CAL, and subgingival (Delta) temperature, were investigated. Each quadrant of the subject’s mouth was assigned to one of three treatments: SRP alone, SRP and local Dox (Atridox™), and local Dox alone. Significant differences were found in favor of SRP with Atridox™ compared with SRP alone and Atridox™ alone [26, 49]. A 6-month clinical investigation revealed no significant enhancement in clinical measures for periodontitis therapy when evaluating the test group (SRP plus Atridox™) to the control group (SRP alone) [28].
The enhancement in PI and GI scores across the four groups is likely attributable to the mechanical debridement, which effectively eliminated, reduced, and minimized periodontal pathogens, coupled with the strict conformity to oral hygiene protocols by each patient. PI and GI were enhanced in participants who underwent SRP + Dox (irrespective of local, systemic, or combined administration) compared to those who received SRP alone. This discovery may be clarified by the antibacterial and anti-inflammatory characteristics of Dox, which reduced the expression of pro-inflammatory mediators and cytokines and limited the activity of polymorphonuclear leukocytes and their scavenging action on reactive oxygen species.
The significant reduction in the PPD and CAL gain in all four groups is likely attributed to the removal of dental plaque and embedded periodontal pathogens from the periodontal pockets, thereby resolving the inflammation and permitting the tissue to heal. The periodontal tissue regenerative properties of Dox comprising anti collagenase effects and promotion of reattachment may justify the reduction in the PPD and CAL gain in subjects who received SRP + Dox (regardless of being local, systemic, or both together) was more than that of subjects who received SRP alone.
In this study, the effect of SRP combined with systemic and local Dox on 15 subjects was assessed over different periods. The results revealed a slight improvement over the other three groups on both clinical and microbiological levels. Several reports indicate the efficacy of the application of two local antibiotics together or the administration of two systemic antibiotics together as adjuncts to SRP in the treatment of periodontitis. Yang, in 2015, tested Minocycline combined with metronidazole both subgingivally. Zhu et al. (2019) and Huang et al. (2021) used a combination of minocycline hydrochloride ointment with Tinidazole [50-52]. Similarly, Cionca et al., 2009; Feres et al., 2018; Winkel et al., 2001 examined the systemic management of amoxicillin together with metronidazole [53-55]. Guzmán et al. tested a mixture of ciprofloxacin and metronidazole [56]. These studies recorded a reduction of pocket depth in all participants during the follow-up periods. The results are corroborated by the conclusions of a systematic review and meta-analysis conducted by Dakic et al. in 2016 [57].
The effective management of periodontal disease requires modifying the presence of periodontal pathogens to eradicate the key agents that trigger the disease, hence promoting tissue healing and the treatment of inflammation [58, 59]. The mechanical removal of periodontal pathogens using scaling and root planing is both necessary and efficient; however, certain forms of the illnesses can linger and require additional medications, such as strong antibiotics. In the current investigation, we utilized CFUs as a microbiological metric [60], analyzing a total of 180 microbiological samples that were obtained from the deepest areas of the pockets. The findings indicated a significant decrease in bacterial counts across all research groups.
Similarly, a 180-day clinical trial in which 30 patients with chronic periodontitis were randomly assigned to 3 groups: SRP alone, SRP + 500 mg of systemic tetracycline twice/day for 14 days, SRP + tetracycline fibers at 4 selected sites for 10 days. Subgingival plaque trials were obtained from 4 designated sites with probing pocket depths of 6 - 10 mm in each subject at baseline, 7, 90, and 180- days post-therapy. The conclusion documented that both the experimental groups showed a significant reduction (p < 0.01) in the incidence of the “red complex” species as linked to the control [61]. The same study with other trials reported that in cases of chronic periodontitis, local and systemic tetracycline therapy significantly minimized the occurrence of around periodontal pathogens and preferred the growth of beneficial species for at least 180 days next to treatment [61-63]. Aligning with these findings, several studies directed in different styles as a large, multi, or single center displayed a bigger decrease in the numbers and extents of red complex bacteria next to the adjunctive utilization of minocycline microspheres [64-67]. The current study showed similar results as the number of the CFUs was reduced at the 45 and 90-day follow-ups. A local study confirmed a related effect on CFUs [35].
Ioannou et al. (2011) studied the control group (SRP with hand instruments) and the experimental group (SRP with piezoelectric device + Atridox™ gel). At the 90-day re-examination, both therapeutic approaches resulted in a statistically significant decrease in the figure of P. gingivalis; no statistically significant reduction was detected for T. forsythia and T. denticola [28].
We recommended further studies with large contributors as well as longer follow-up periods. A double-blinded study and or/ randomized clinical trials are highly recommended to reduce the risk of bias. A “checkerboard” DNA-DNA hybridization or PCR technique is more precise for the microbiological analysis of the microorganisms associated with periodontitis. The number of participants, a single center, and a single-blinded study were considered as limitations.
CONCLUSION
In lieu of the above findings, the present study concludes that all the different treatment modalities improved the clinical and microbiological parameters of patients with mild or moderate periodontitis after 45 and 90 days. SRP with doxycycline provided more improvement in clinical and microbiological parameters than that of SRP alone. Using SRP with systemic plus local Dox (both together) exhibits a superior effect over using SRP alone and SRP with either systemic or local doxycycline alone.
AUTHORS’ CONTRIBUTIONS
The authors confirm their contribution to the paper as follows: W.A.A.H.: Study conception and design; D.M.M.A.: Conceptualization; H.E.H.: Investigation; A.S.A.S., M.M.A.H., M.M.M.A., M.M.A.M., V.M.: Draft manuscript. All authors reviewed the results and approved the final version of the manuscript.
LIST OF ABBREVIATIONS
| SRP | = Scaling and Root Planning |
| Dox | = Doxycycline |
| PI | = Plaque Index |
| GI | = Gingival Index |
| PPD | = Probing Pocket Depth |
| CAL | = Clinical Attachment Level |
| CFUs | = Colony Forming Units |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The Medical Ethical Committee of the Faculty of Medicine, Sana'a University, Yemen provided ethical approval (ref = 143 / Dec 2, 2023).
HUMAN AND ANIMAL RIGHTS
All procedures performed in studies involving human participants were in accordance with the ethical standards of institutional and/or research committees and with the 1975 Declaration of Helsinki, as revised in 2013.
CONSENT FOR PUBLICATION
All subjects signed informed consent prior to any intervention, following a thorough description of each treatment procedure and potential risks.
AVAILABILITY OF DATA AND MATERIALS
The data supporting the findings of the article will be available from the corresponding author [M.M.Al.M] upon reasonable request.
ACKNOWLEDGEMENTS
Declared none.


